Section 16.2 heat and thermodynamics – Section 16.2: Heat and Thermodynamics delves into the fascinating realm of energy transfer and its impact on matter and the universe. From the intricacies of heat transfer to the fundamental principles of thermodynamics, this section unveils the secrets that govern the behavior of energy in our world.
Heat transfer, the movement of thermal energy from one object to another, plays a pivotal role in various industries and everyday life. Thermodynamics, on the other hand, provides a framework for understanding the interconversion of heat and other forms of energy, with its laws guiding the direction and efficiency of energy transformations.
Heat Transfer

Heat transfer is the movement of thermal energy from one object or region to another. It occurs when there is a temperature difference between the two objects or regions. Heat transfer can occur through three main modes: conduction, convection, and radiation.
Conduction, Section 16.2 heat and thermodynamics
- Conduction is the transfer of heat through direct contact between two objects.
- For example, when you touch a hot stove, heat from the stove is transferred to your hand through conduction.
- The rate of heat transfer by conduction depends on the temperature difference between the two objects, the area of contact, and the material of the objects.
Convection
- Convection is the transfer of heat through the movement of a fluid (liquid or gas).
- For example, when you boil water, heat from the bottom of the pot is transferred to the water through convection.
- The rate of heat transfer by convection depends on the temperature difference between the fluid and the surrounding environment, the velocity of the fluid, and the density of the fluid.
Radiation
- Radiation is the transfer of heat through electromagnetic waves.
- For example, heat from the sun is transferred to the Earth through radiation.
- The rate of heat transfer by radiation depends on the temperature of the radiating object, the emissivity of the object, and the distance between the object and the receiving object.
Thermodynamics: Section 16.2 Heat And Thermodynamics
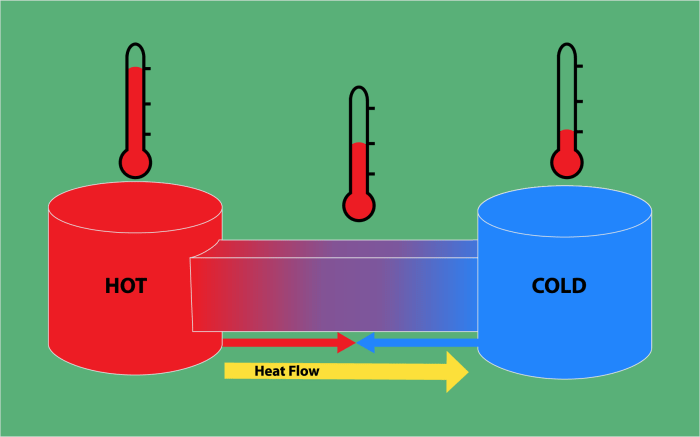
Thermodynamics is the branch of physics that deals with the relationships between heat and other forms of energy.
First Law of Thermodynamics
The first law of thermodynamics states that energy cannot be created or destroyed, only transferred or transformed.
Second Law of Thermodynamics
The second law of thermodynamics states that the entropy of a closed system always increases over time.
Applications of Heat and Thermodynamics

Heat and thermodynamics have a wide range of applications in various industries and everyday life.
Applications of Heat Transfer
- Heat transfer is used in the design of heating and cooling systems for buildings.
- Heat transfer is used in the food industry to preserve food.
- Heat transfer is used in the manufacturing of electronic devices.
Applications of Thermodynamics
- Thermodynamics is used in the design of engines and other devices that convert heat into mechanical energy.
- Thermodynamics is used in the design of refrigeration and air conditioning systems.
- Thermodynamics is used in the study of weather patterns and climate change.
Heat and Thermodynamics in Nature
Heat and thermodynamics play a crucial role in various natural phenomena.
Role of Heat and Thermodynamics in Weather Patterns
Heat transfer and thermodynamics drive the formation of clouds, precipitation, and wind patterns.
Role of Heat and Thermodynamics in the Formation of Stars and Planets
The gravitational collapse of gas and dust clouds leads to the formation of stars and planets, releasing immense amounts of heat and energy.
Role of Heat and Thermodynamics in the Behavior of Animals and Plants
Animals and plants have evolved various adaptations to regulate their body temperature and respond to changes in their thermal environment.
Key Questions Answered
What are the three modes of heat transfer?
Conduction, convection, and radiation.
What is the first law of thermodynamics?
Energy cannot be created or destroyed, only transferred or transformed.
What is the second law of thermodynamics?
The entropy of an isolated system always increases over time.